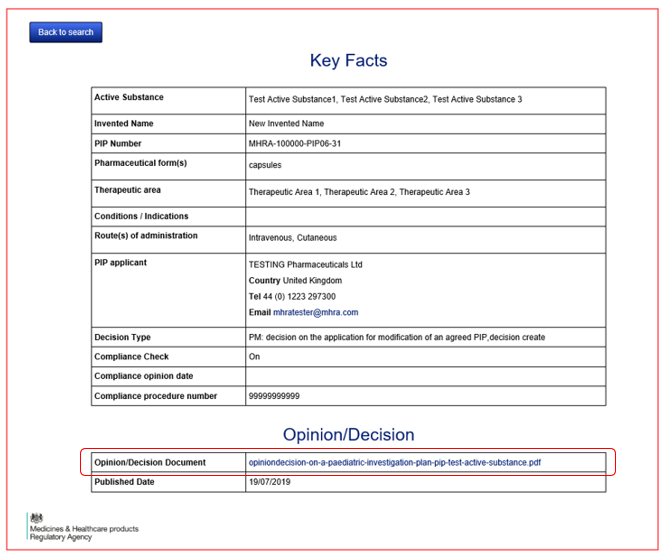
Yes, when you click on the document hyperlink to display the full details of the PIP, you will be able to click on the hyperlink of the Opinion/Decision Document, open the PDF and download or print it from there.
Alpha Release This is a new service - your feedback will help improve it.
Yes, when you click on the document hyperlink to display the full details of the PIP, you will be able to click on the hyperlink of the Opinion/Decision Document, open the PDF and download or print it from there.

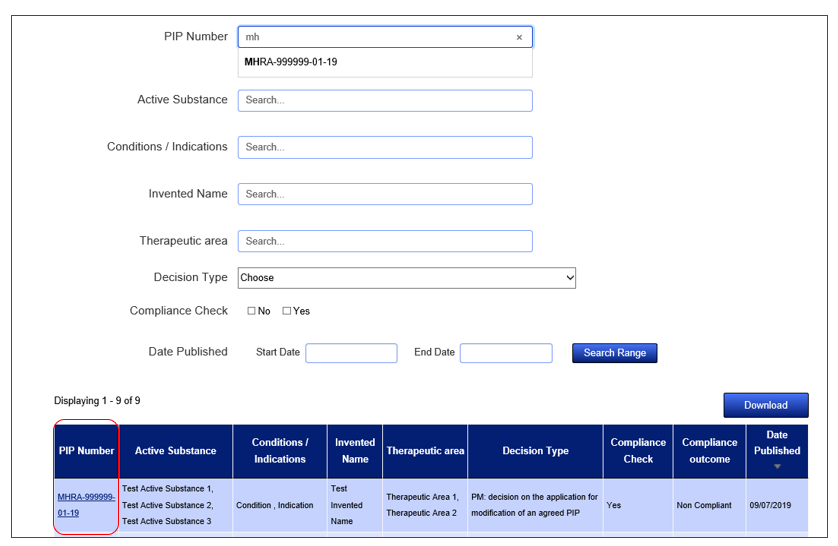
After you have identified the PIP you wish to find more information on, click on the hyperlink that is presented in the column ‘PIP Number’, in the left-hand column of the results table. This link will bring you to the ‘Key Facts’ page.
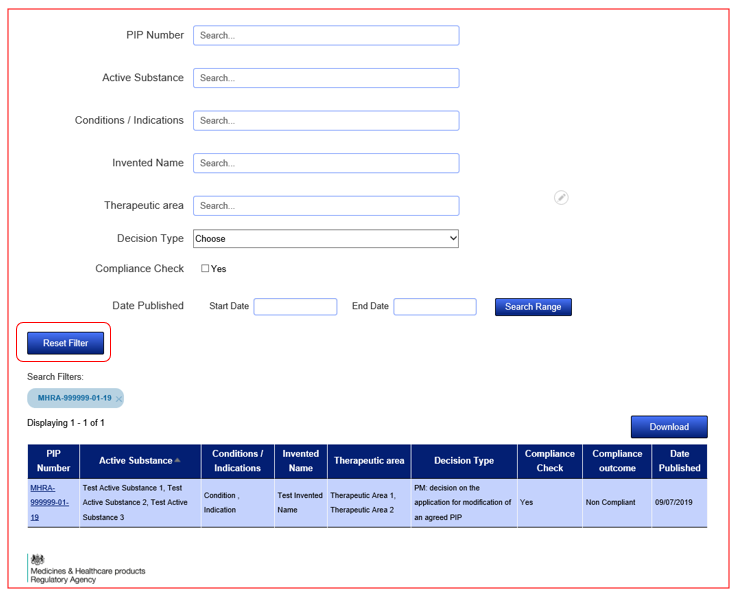
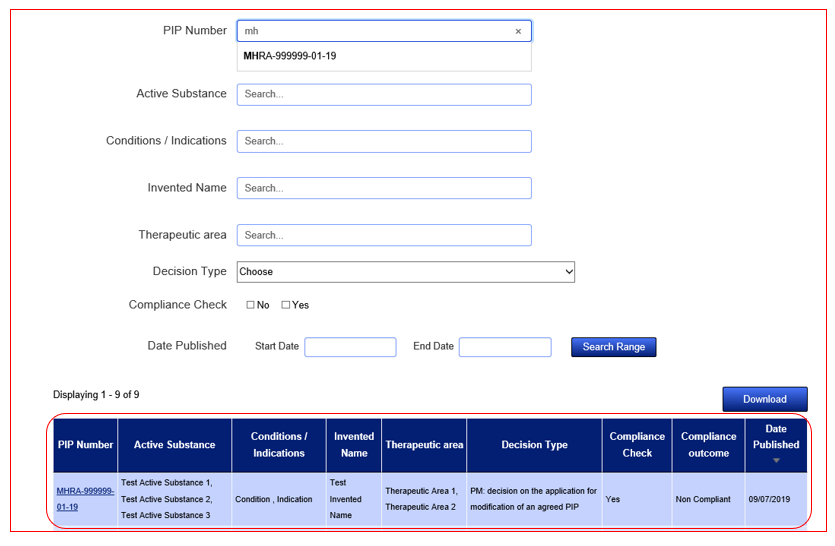
Yes, this can be done by clicking the ‘Download’ button found at the top right of your results.
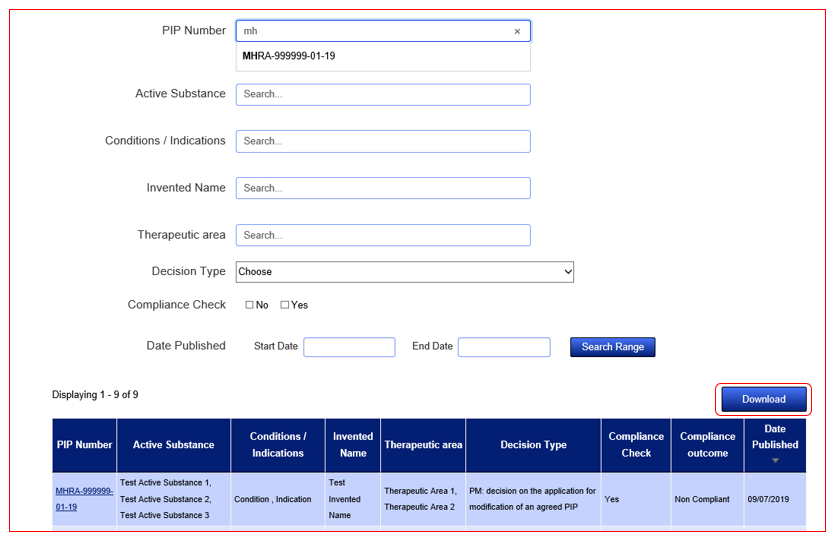
Search fields are dynamic and will auto populate with suggestive entries once you start typing. Simply select the entry that applies to your search from the dropdown list and the results will refresh in the tabular view below the search fields. You can narrow your search results by using the Active Substance field to search for a combination of Active Substances related to one PIP Number. You can widen your search through the use of the remaining search fields. Make sure to use the 'Reset Filter' feature if you wish to start over.
To then view the details of the document, click on the hyperlink PIP Number and the full details will appear.

PIP outcome documents published before exit day are available from the EMA website. PIP opinion documents published by the MHRA after exit day are available on the MHRA website here.
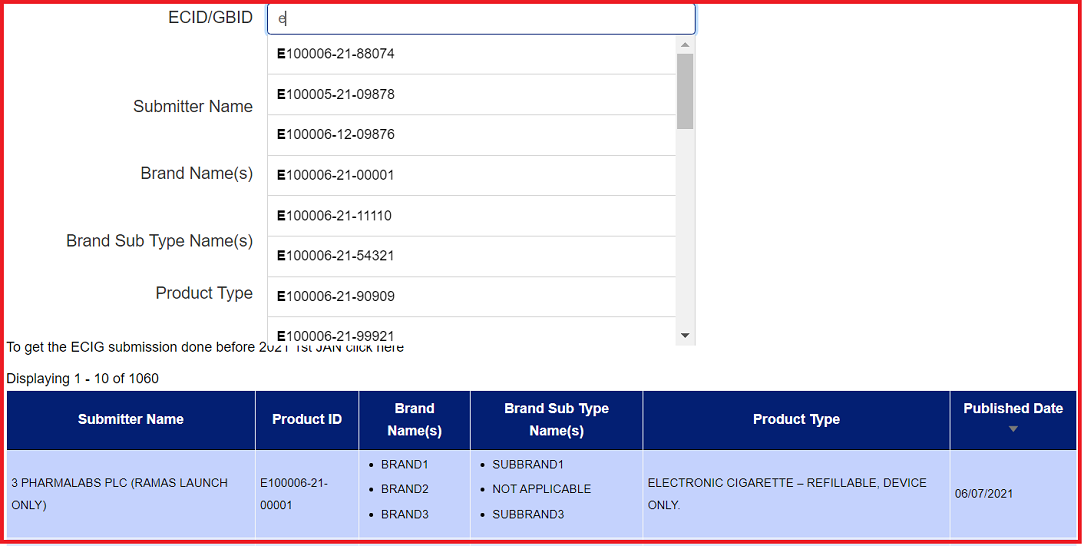
Search fields are dynamic and will auto populate with suggestive entries once you start typing. Simply select the entry that applies to your search from the dropdown list and the results will refresh in the tabular view below the search fields. You can narrow your search results by using the Submitter Name field to search for a combination of Submitter Name related to one ECID/GBID. You can widen your search through the use of the remaining search fields. Make sure to use the 'Reset Filter' feature if you wish to start over.
Previously notified are still legal for supply and published. Details of these products will appear in the historic links available on the main publication page.
To download the excel file, Click on the respective link under the Excel to Download column.

The product may have been published prior to January 2021 and may appear in one of the links provided on the main publication page. If the product you are looking for is not published, it cannot be legally suppled in the UK.

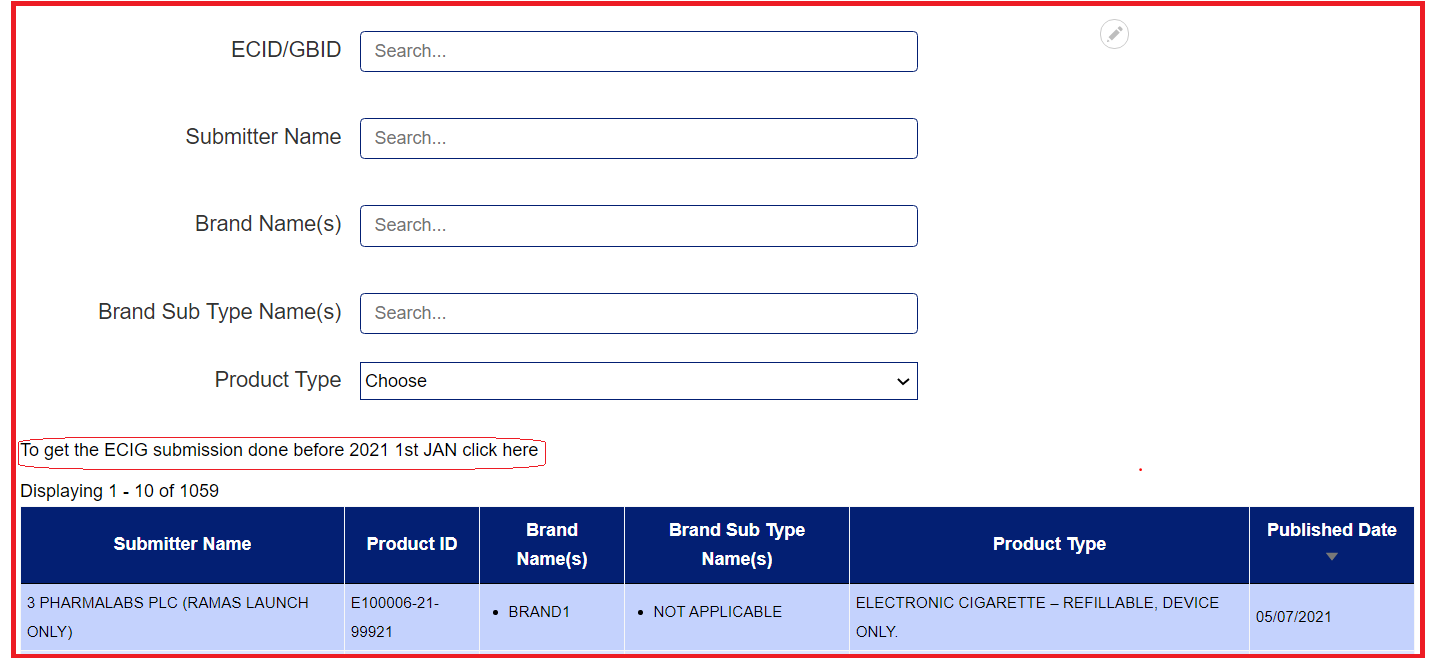
Once you have made a search query, a 'Reset Filter' button will appear. Click on this to reset your search query to start over.
Reporting an adverse reaction via Yellow Card
If you feel unwell after using an e-cigarettes product you can report side effects and safety concerns with e-cigarettes or refill containers to the MHRA through the Yellow Card scheme.
If you would like to provide information about the sale of defective, non-compliant or illegal e-cigarette products you can contact the MHRA at TPDsafety@mhra.gov.uk, alternatively you can contact Trading Standards.
Detailed information and guidance on E-cigarette products can be found on our main MHRA page here.
The data submitted in notifications is reviewed by the MHRA under Regulation 31 (Notification about electronic cigarettes and refill containers) of the Tobacco and Related Products Regulations 2016 (TRPR).
Once your submission has been published to the relevant country list (GB or NI) the product is deemed to have complied with the notification requirements
Note: Publication of a submission demonstrates only that compliance with Regulation 31 has been achieved. Should a product fail to comply with further requirements of Part 6 of TRPR, or related legislation it could be subject to seizure by UK enforcement agencies or withdrawal/recall from the market.
If you feel that the information on the system is incorrect, then please contact tpdnotifications@mhra.gov.uk for assistance. Please give as much information as possible to help the team investigate the discrepancy.
In order to wholesale distribute medicinal products organizations must be in possession of a wholesale dealers authorization and fulfil certain obligations, one of which is to comply with the principles and guidelines of good distribution practice. When medicinal products are supplied or received a check to ensure the appropriate authorization is held needs to be performed. In addition where medicinal products are received from another wholesaler the receiving wholesaler must verify that the supplier complies with the principles and guidelines of good distribution practice. Part of this check is to ascertain if the supplier holds a GDP certificate
MHRA endeavor to perform re-inspections in advance of the validity date of the GDP certificate in order to ensure continuity of the documentation. However due to the risk based prioritization of resources this is not always possible and we are aware that some GDP certificates have now passed their validity date.
Where a sites GDP certificate has passed its validity date wholesalers should continue to perform their own checks to assure themselves that an appropriate authorization is held and the principles and GDP guidelines are being adhered to.
Checks to verify the supplier complies with GDP could include:
1. If they have more than one site, then reference to GDP certificates issued to other sites more recently
2. For established suppliers, a review of deviations / complaints / damages / returns records relating to the supplier
3. For new suppliers a declaration of GDP compliance provided by the RP
4. Evidence that a recent inspection has taken place which has yet to generate a new certificate.
It can take 2 days for your Authorisation or Certificate to appear in MHRA-GMDP. If this is not the case, then please contact info@mhra.gov.uk for assistance, giving as much information as possible to help the team answer your query.
For queries concerning the information displayed in MHRA-GMDP please contact info@mhra.gov.uk for assistance, giving as much information as possible to help the team answer your query.
When carrying out a search, the results are refreshed in the table below.
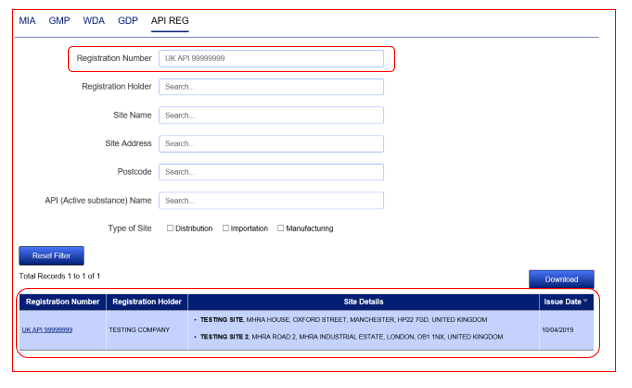
It is then possible to carry out a further search without losing the results of the previous search. To do this, proceed to perform a further search without clicking the ‘Reset Filter’ button. 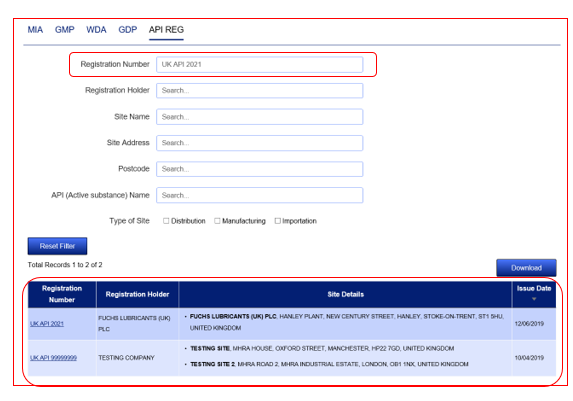
This will then mean that the second set of results will appear alongside the 1st query results allowing you to see your 2 results together on the same screen. You can repeat this process to add further results.
When searching within MHRA-GMDP there is no specific search button to select. Instead, the search fields displayed are dynamic and will auto populate with suggestions once you start to type information into them. For example, if you type 999 into the Authorisation Number field the available entries will appear as a drop down.
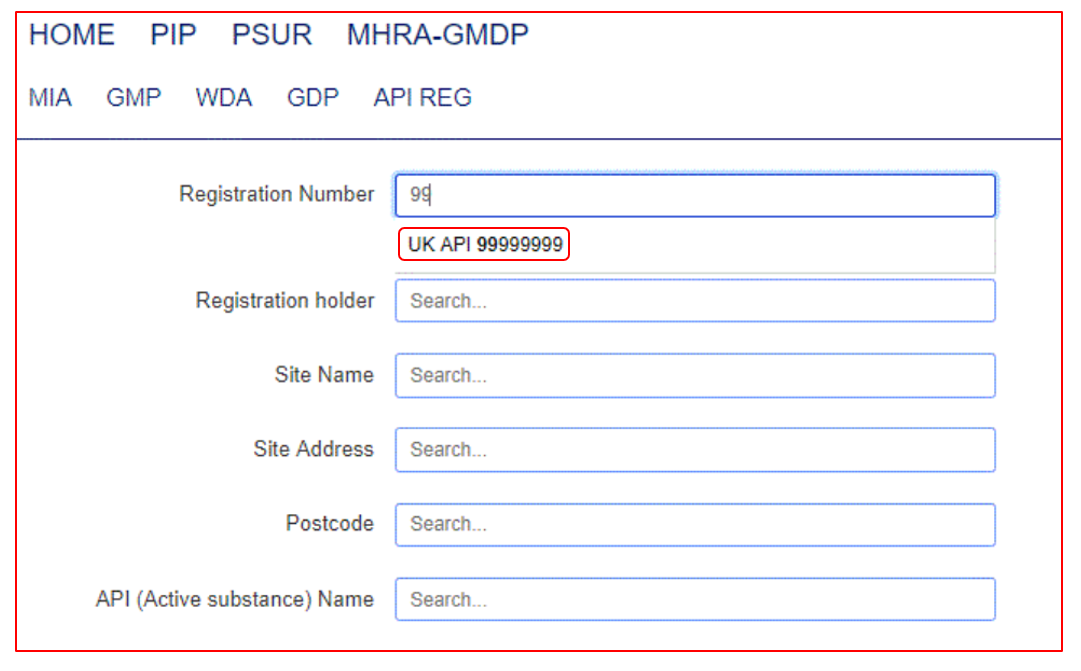
When you are ready to see the results of the search, simply click out of the search field and the query will then run, and the results refreshed below.
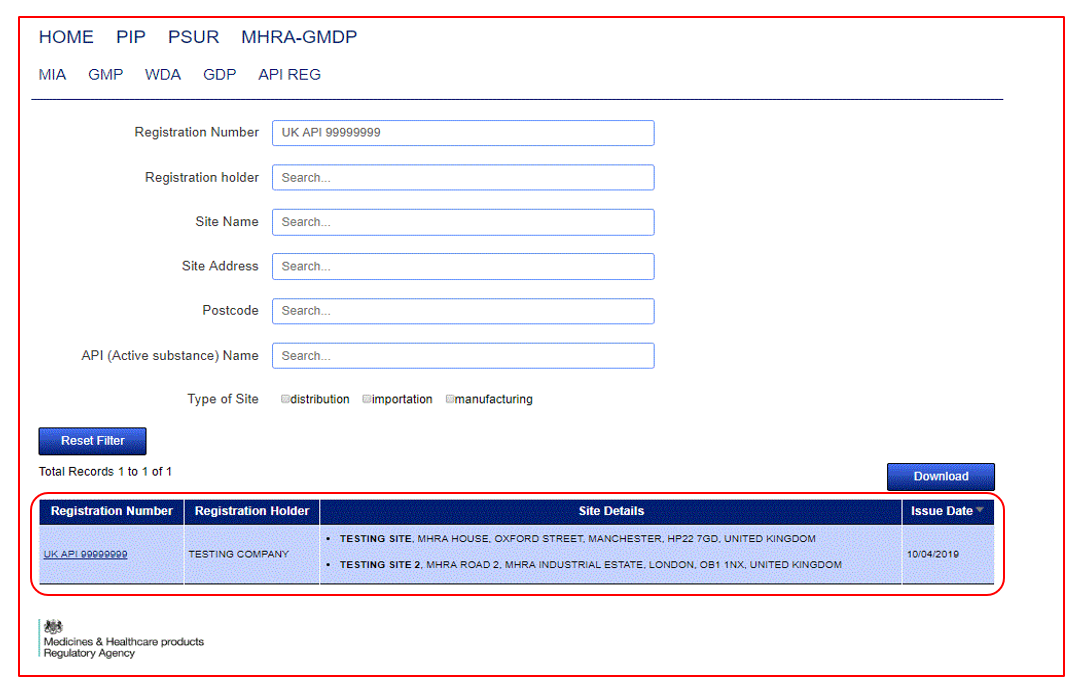
To then view the details of the document, click on the hyperlink and the full details will appear.
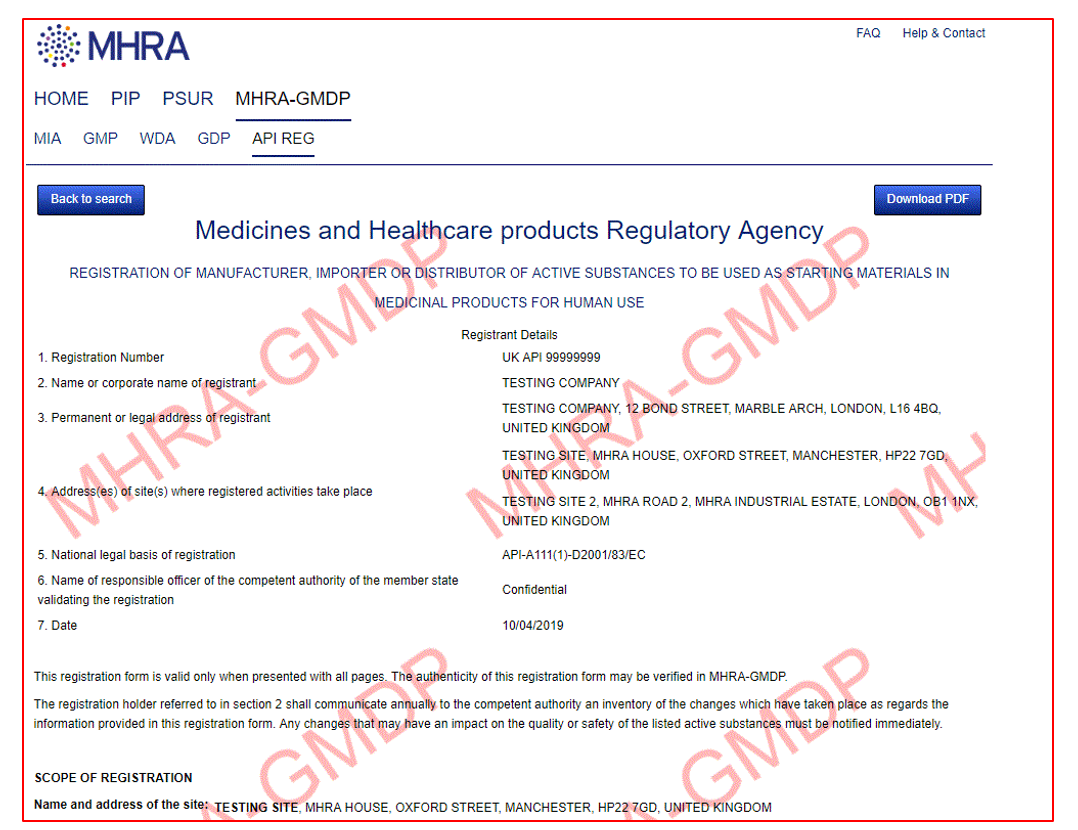
Yes, when you click on the document hyperlink to display the full details of the Authorisation/Certificate a download PDF button will be visible. Click the download PDF button and the document will be converted to pdf which will then allow you to print. This will be watermarked ‘MHRA-GMDP’ to make it clear that it’s a copy of the original document.
Please allow 2 working days from when your document was issued to then appear on the MHRA-GMDP system. If after this time period has elapsed it is still not present, please contact info@mhra.gov.uk for assistance, giving as much information as possible to help the team answer your query.
If you feel that the information on the system is incorrect, then please contact info@mhra.gov.uk for assistance. This could be the company name, address, site functions, dates or other details. Please give as much information as possible to help the team investigate the discrepancy.
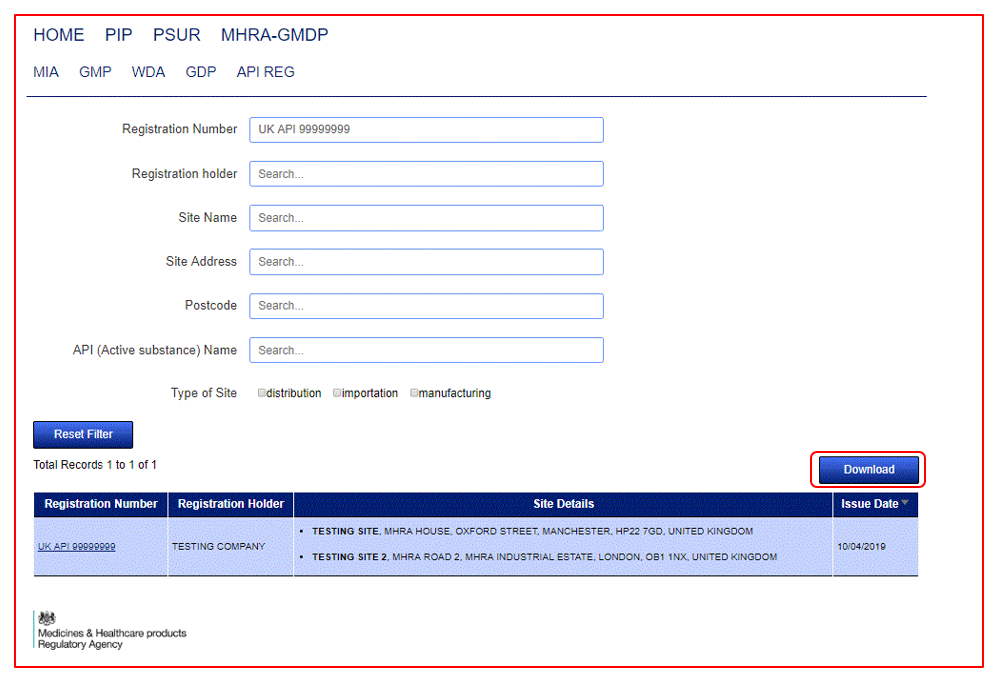
Yes, this can be done by clicking on the Download button found at the top right of the results.
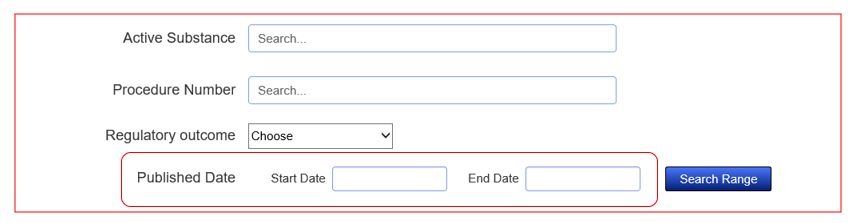
Yes, you can search for records between certain dates and based on the start or end date. To do so look to the bottom of the search fields at ‘Date Published’. Input the specific dates you wish to search for, remembering to put them in the correct order.